Iron Iii Chloride and Sodium Hydroxide Ionic Equation
1 Here is the complete ionic equation. The net ionic equation is.

How To Write The Net Ionic Equation For Fecl3 Naoh Fe Oh 3 Nacl Youtube
Double replacement Please tell about this free chemistry software to your friends.
. Hence it deposits as a precipitate at the bottom of the reaction. IronIIIchloride sodium hydroxide. FeCl3 reacts with NaOH to form Fe OH3 and NaCl.
Therefore it exists as an aqueous solution and NaOH also exists as a solution. 3 33NaOH FeOH 3 NaCl FeOH 3. Fe3 3OH-1 3Na1 3NO3-1 ------ Fe OH3 3NaNO3.
When ammonia which has a chemical formula of NH3 dissolves in water chemical formula H2O it forms a solution of ammonium hydroxide NH4OH. Fe 3 aq 3 OH-aq FeOH 3 s This is because three hydroxide ions are needed to. If you get a green precipitate the unknown substance is ironII.
3NaOH FeCl 3 Fe OH 3 3NaCl. Since iron III chloride and sodium chloride are soluble in water but not iron III hydroxide the reaction causes a solid to be precipitated. As iron III hydroxide is brown a brown precipitate is formed.
Fe NO33 3NaOH ------ Fe OH3 3NaNO3. But solubility of Fe OH 3 is poor in water. Direct link to this balanced equation.
Sodium hydroxide react with iron III chloride to produce iron III hydroxide and sodium chloride. The balanced equation for the reaction is. The ionic equation is Fe22OH-Fe OH2 then it will be oxidated to Fe OH3.
If playback doesnt begin shortly try restarting your device. Observations Full equation Precipitate formed Net ionic equation orange precipitate forms FeCl. Specific Reactions Iron III chloride Sodium hydroxide Observation Molecular Equation Ionic Equation Net lonic Equation Driving Force Iron III chloride Copper ll sulfate Observation Molecular Equation lonic Equation Netlonic Equation Driving Force fron III chloride Potassium phosphate Observation Molecular Equation Sonic.
100 4 ratings FeCl3 aq 3 NaOH aq --- Fe OH3 s 3 NaCl aq B. A dictionary entry for the word castigation from merriam-webster. Fe³ Cl NH₄ 3 OH -- FeOH₃s NH₄ Cl.
Iron III chloride sodium hydroxide iron III hydroxide sodium chloride. If you have done this double check that you and your pair yesterday have the correct formula written. B Complete the total ionic equation for the reaction of aqueous ironIII chloride with aqueous sodium hydroxide.
What is the molecular ionic and net ionic equations for iron III chloride sodium hydroxide. FeCI3 3NaOH -FeOH3 3NaCIIron III chloride Sodium Hydroxide --- iron III hydroxide sodium chloride. To find the complete ionic equation dissociate the compounds in aqueous phases into their ionic forms.
Ironiii chloride Sodium hydroxide Observation Color change precipitate Molecular Equation FeCl3aq 3NaOHaqFeOH3s 3NaClaq Ionic equation Fe 3Cl-3Na 3OH-FeOH 3 3Na3Cl- Net Ionic Equation Fe 3OH-FeOH 3. IronIIIChloride SodiumHydroxide IronIIIHydroxide SodiumChloride Reaction type. In the precipitation reaction which identifies the ironIII ion as being present the ionic equation is.
FeCl₃ aq 3 NH₄OH aq FeOH₃ s 3 NH₄Cl aq The precipitate is FeOH₃ or iron iii hydroxide. See the answer See the answer done loading. The balanced equation would be.
Aston villa contact number. Videos you watch may be added to the TVs watch history and influence TV recommendations. In other words iron III chloride reacts with sodium hydroxide to form iron III hydroxide and sodium chloride.
This is the best answer based on feedback and ratings. As iron III hydroxide is brown a brown precipitate is formed. Remember to include the states of matter for the products.
Fe3 aq 3Cl aq 3Na aq 3OH aq. Death knight 5e monster manual. The balanced equation for the reaction between FeCl3 and NaOH is FeCl3 3NaOH - Fe OH3 3NaCl.
NaOH ------ Na 1 OH -1. F eCl3aq 3N aOH aq F eOH3s 3N aClaq. Black espadrilles wedges.
FeCl 3 aq 3NaOH aq Fe OH 3 s 3NaCl aq FeCl 3 is soluble in water. To tell whether an unknown substance contains ironII nitrate or ironIII nitrate add a few drops of sodium hydroxide solution. The ionic equation is Fe 2 aq 2OH-aq FeOH 2 s I ron II hydroxide is green.
2K aq 2Al 3 aq 8OH - aq 2H aq SO 42- aq --- 2Al OH 3 s 2K aq SO 42- aq 2H 2 O ℓ Note that the sulfuric acid is treated as fully dissociated. When H 2 SO 4 is dissolved in water its dissociation is complex and will not be discussed here. View the full answer.
Complete your lab worksheet from yesterday filling in the top three boxes for all reactions.

Solved A Balance The Reaction Of Sodium Hydroxide And Chegg Com

How To Balance Naoh Fecl3 Fe Oh 3 Nacl Sodium Hydroxide Iron Iii Chloride Youtube
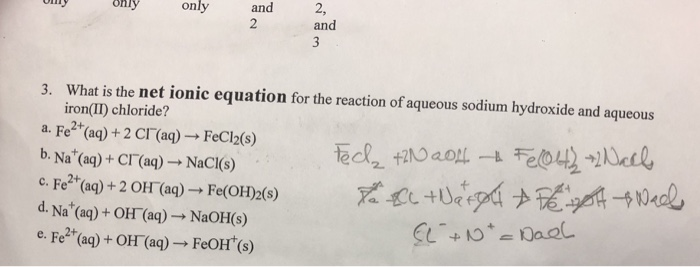
Solved H Only And 2 2 And 3 What Is The Net Ionic Equation Chegg Com
No comments for "Iron Iii Chloride and Sodium Hydroxide Ionic Equation"
Post a Comment